LOW DOSE NALTREXONE
What is Low Dose Naltrexone (LDN)?
Naltrexone was synthesized in 1963 as an orally active competitive opioid receptor antagonist. Naltrexone was approved by the FDA in 1984 for the treatment of opioid addiction. LDN refers to daily dosages of naltrexone that are approximately 1/10th of the typical opioid addiction treatment dosage.
Naltrexone blocks opiate drugs from binding to the opioid receptors, which can result in increased endorphin and enkephalin release. This results in reduced:
- Signaling and release of inflammatory substances,
- Nerve cell inflammation, and
- Autoimmune mediators.
Which patients may benefit
from LDN therapy?
- Allergies and Asthma
- Alzheimer’s
- Arthritis
- Autism Spectrum Disorder
- Autoimmune Disorders
- Cancer
- Celiac Disease
- Complex Regional Pain Syndrome (CRPS)
- Chronic Fatigue and Fibromyalgia
- Chronic Pain
- Chronic Pruritus
- Crohn’s and Ulcerative Colitis
- Diabetic Neuropathy
- Eczema
- Grave’s Disease
- Hashimoto’s Thyroiditis
- HIV/Aids
- Lupus
- Lyme Disease
- Mood Disorders
- Multiple Sclerosis
- Pain and Inflammation
- Parkinson’s
- Psoriasis
- Sjogren’s Syndrome
- Traumatic Brain Injury (TBI)
LDN can be administered
in any of these forms:
- Capsules
- Liquid
- Sublingual Drops
- Topical Cream
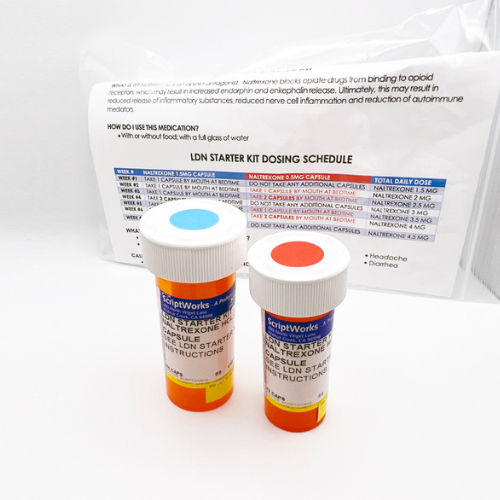
Low Dose Naltrexone (LDN) Starter Kit from ScriptWorks
Dosing schedule to properly titrate for optimal patient outcomes.