Author: Bob Brensel | President, Pharmacist | ScriptWorks
Bob Brensel, RPh, earned his Pharmacy Degree at University of the Pacific in Stockton, California in 1980. Former California Pharmacists Association’s Award Winner for Recognition of Outstanding Achievement in Compounding Pharmacy. Read More →
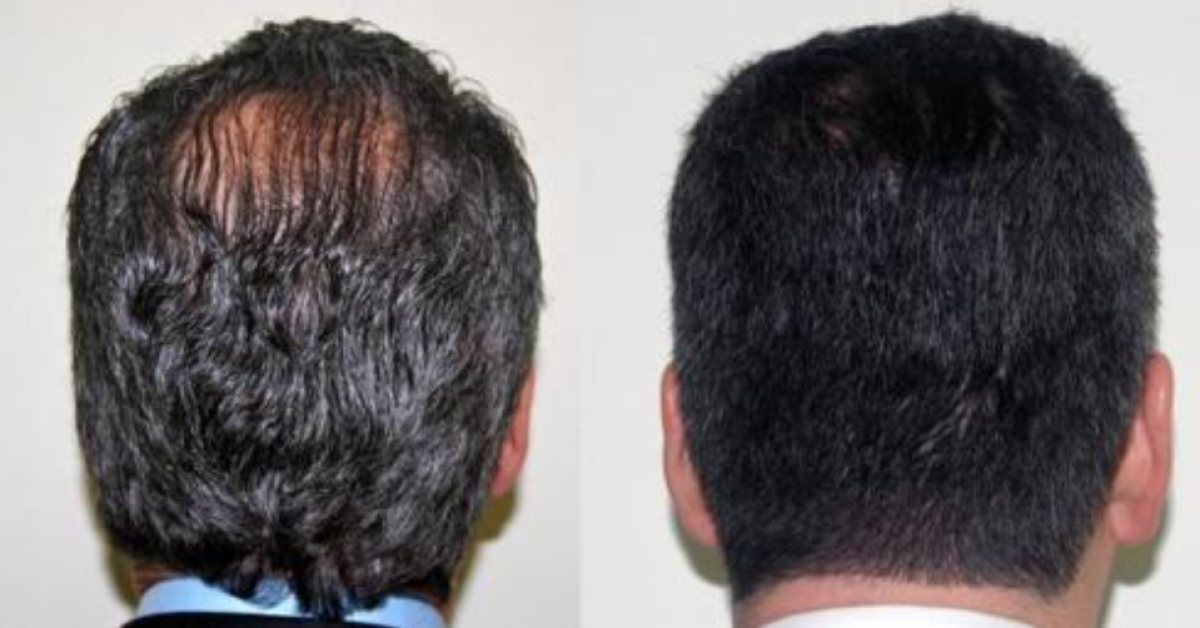
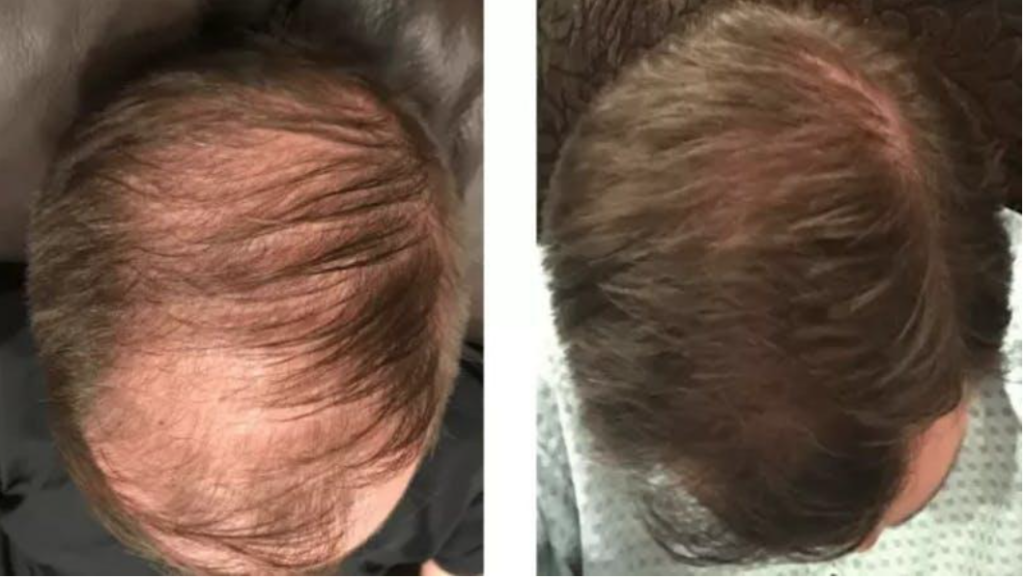
Baldness (androgenic alopecia) is the most common type of hair loss. It affects an estimated 80 million individuals in the U.S. Source : Cleveland Clinic
One of the hottest topics in pharmacy compounding these days is hair restoration. Well, ScriptWorks collaborates with many hair restoration doctors in the Bay Area to create customized formulas that help both men and women with hair loss problems.
Oral & Topical Therapies through Compounding
There are so many exciting formulas that are both oral and topical that are therapy options for both men’s and women’s hair loss. Let’s review some of the possibilities.
Common formulas for hair restoration:
MINOXIDIL 0.625mg, 0.25mg CAPSULES
MINOXIDIL 5%/TRETINOIN 0.01%/ FLUOCINOLONE 0.01% TOPICAL SOLUTION
MINOXIDIL 7%/FINASTERIDE 0.1% (PROPYLENE GLYCOL FREE) TOPICAL LOTION
MINOXIDIL 10%/FINASTERIDE 0.2%/TRETINOIN 0.025% TOPICAL CREAM
Minoxidil Sulfate & Finasteride
The gold standard is using Minoxidil in hair growth formulas and the goal is to get the best absorption into the skin possible without causing significant patient side effects.
Minoxidil Sulfate is an active metabolite which stimulates hair growth according to many studies. It is the number one product used for Androgenic Alopecia and has been approved by the FDA for this indication.
Finasteride is a 5 alpha-reductase inhibitor that blocks the conversion of testosterone to dihydrotestosterone (DHT), the androgen responsible for male pattern hair loss (androgenic alopecia). Finasteride is FDA approved for androgenic alopecia in men.
At ScriptWorks, we often tailor specific formulas containing one or both or these ingredients.
ScriptWorks has many other options for hair growth including Low Dose Oral Minoxidil. There are many other drugs like Tretinoin, Spironolactone, Fluocinonide, and others that doctors can use to enhance each compounded formula.
ScriptWorks’ pharmacists primary job is to research the safety and efficacy of our compounded drug therapies before creating a customized prescription for our patients. Our goal is to create a safe, patient-specific dosage form.
Before we filled our first compound for Oral Minoxidil Therapy, we did an in-depth analysis of the safety and side effects of Low Dose Oral Minoxidil. We found quite a few studies (see below) that observed positive results of low dose oral Minoxidil. Since then, more studies have come to the forefront of this therapy. There are side effects that need to be considered and weighed against potential benefits.
A total of 17 studies with 634 patients were evaluated in consideration of oral Minoxidil therapy for hair loss. The conclusion was that oral minoxidil was found to be a well-tolerated treatment alternative for healthy patients having difficulty with topical formulations.
An Old Medicine Grows New Hair for Pennies a Day, Doctors Say
by Gina Kolata, The New York Times
Dermatologists who specialize in hair loss say that the key ingredient in a topical treatment worked even better when taken orally at a low dose.
Source: The New York Times
This article has led to many inquiries from doctors and patients about this therapy.
Are you a Hair Restoration Doctor or Specialist?
Let’s connect, to better approach your patients’ specific needs.
Our expert pharmacists work with specialists in Walnut Creek, Concord, Martinez, San Francisco, Oakland, Berkeley, Danville, Lafayette, Alamo, & More!
Contact your local dermatologist or hair restoration specialist about whether a topical or oral compounded form of Minoxidil with other ingredients is right for you.
Already have a prescription for your hair restoration and not sure where to fill it? Come to the experts. Easily fill your prescription with ScriptWorks >>
You can also call and speak to a ScriptWorks pharmacist to help you get started on your hair restoration journey.
More Articles:
Randolph M, Tosti A.J Am Acad Dermatol. 2021 Mar;84(3):737-746. doi: 10.1016/j.jaad.2020.06.1009. Epub 2020 Jul 2.PMID: 32622136 Review.
Beach RA, McDonald KA, Muylaert Barrett B.J Am Acad Dermatol. 2021 Mar;84(3):761-763. doi: 10.1016/j.jaad.2020.10.032. Epub 2020 Oct 22.PMID: 33098962 No abstract available.
Sharma AN, Michelle L, Juhasz M, Muller Ramos P, Atanaskova Mesinkovska N.Int J Dermatol. 2020 Aug;59(8):1013-1019. doi: 10.1111/ijd.14933. Epub 2020 Jun 9.PMID: 32516434
Jha AK, Sonthalia S, Zeeshan MD, Vinay K.J Am Acad Dermatol. 2020 Nov;83(5):1491-1493. doi: 10.1016/j.jaad.2020.05.129. Epub 2020 May 31.PMID: 32492469 No abstract available.
Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S.J Am Acad Dermatol. 2019 Aug;81(2):648-649. doi: 10.1016/j.jaad.2019.04.054. Epub 2019 May 2.PMID: 31054970 No abstract available.
Bammel A, Stern R, Sterry W.Dtsch Med Wochenschr. 1993 May 28;118(21):787-9. doi: 10.1055/s-2008-1059391.PMID: 8504714 Review. German. No abstract available.
Stoehr JR, Choi JN, Colavincenzo M, Vanderweil S.Am J Clin Dermatol. 2019 Apr;20(2):237-250. doi: 10.1007/s40257-018-0409-y.PMID: 30604379 Review.
Ramírez-Marín HA, Tosti A.Indian Dermatol Online J. 2022 Oct 12;13(6):729-733. doi: 10.4103/idoj.idoj_246_22. eCollection 2022 Nov-Dec.PMID: 36386734 Free PMC article. Review.
Gupta AK, Hall DC, Talukder M, Bamimore MA.Skin Appendage Disord. 2022 Sep;8(5):355-361. doi: 10.1159/000525137. Epub 2022 Jun 30.PMID: 36161084 Free PMC article.